Treatment Of Wet AMD
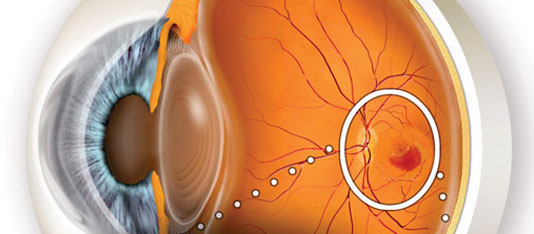
Wet age-related macular degeneration (Wet AMD) describes a condition whereby abnormal blood vessels grow under the central retina, or macula, inside of the eye. It can cause severe and sometimes rapid loss of central vision if left untreated. Wet AMD arises as a complication of Dry age-related macular degeneration (or Dry AMD). Your Retina Doctor may sometimes refer to Wet AMD as “bleeding inside of your eye,” “bleeding under the retina,” “leakage inside of your eye or retina,” or “fluid under the retina.”
Treatment of Wet AMD is aimed at suppressing the vision damaging effects of the disease. As of yet however there is no cure for AMD. With successful treatment of Wet AMD, vision can be maintained for a longer period of time than it otherwise would have been if no treatment was administered. In some patients with recent vision loss from Wet AMD, vision may improve with treatment1, 2, 3. It is important to understand though that treatment of Wet AMD is a life-long regimen of disease suppressing therapy.
Currently, Wet AMD is treated by injecting medicine directly into the eye in a procedure performed right in the doctor’s office. The eye is made numb with eye drops prior to treatment so minimal discomfort is felt during the procedure which takes just a few seconds. Injections may have to be given regularly, as frequently as once every month, perhaps indefinitely, in order to maintain the long term benefits of treatment.
Learn more about the Treatment Of Wet AMD
Side effects of treatment include (but may not be limited to):
- Temporary eye pain after the treatment – this may range from a “sandy” or “scratchy” sensation to “burning” or “stinging”
- Red eye – this may appear quite dramatic and the eye may remain “blood shot” for up to 2 weeks in some cases
- Floaters – “spots” may be seen in the eye after the treatment and usually subside within a few days
- Blurred vision – usually subsides after a day
Risks of treatment are very rare and include (but may not be limited to):
- Permanent loss of vision
- Blood clots causing stroke or heart attack (very rare)
Treatment options:
There are currently three drugs currently FDA approved for the treatment of wet macular degeneration: Lucentis, Eylea, and Beovu. Avastin is another drug that is commonly used to treat wet macular degeneration that is currently used “off-label.” That is, it does not have FDA approval for use to treat Wet AMD. Many drugs used routinely today are used “off label” at a doctor’s discretion. Aspirin, for example, is commonly used to reduce the risk of heart attack or stroke, and yet was never FDA approved for that purpose.
Another option that some of our patients choose is to enroll in a clinical trial in order to potentially access new drugs currently being developed for wet macular degeneration4. See our clinical trials page for currently enrolling trials.
Dosing of treatment:
Injections to the eye are administered on an on-going basis. Clinical trials have shown that greatest benefit is achieved with monthly injections, at least initially. In some cases, the frequency of dosing can be subsequently reduced to every 2 months or longer depending on the individual patient’s response to treatment.
The treatment of Wet AMD is evolving. There are many research studies called clinical trials that are currently underway investigating new medications or different strategies using existing treatments for Wet AMD that may prove to be better than the existing treatments4. Your doctors at Retinal Consultants are proud to be involved in some of these studies and there may be opportunities for you to participate. Ask your Retina Doctor if you would be a candidate for a clinical trial.
References
- Chang MA, Do DV, Bressler SB, Cassard SD, Gower EW, Bressler NM. Prospective One-Year Study of Ranibizumab for Predominantly Hemorrhagic Choroidal Neovascular Lesions in Age-Related Macular Degeneration, Retina: September 2010;30(8):1171-1176. journals.lww.com/retinajournal/Abstract/2010/09000
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006 Oct 5;355(14):1419-31. nejm.org/doi/full/10.1056/nejmoa054481
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012 Dec;119(12):2537-48. doi: 10.1016/j.ophtha.2012.09.006. Epub 2012 Oct 17. Erratum in: Ophthalmology. 2013 Jan;120(1):209-10. pubmed.ncbi.nlm.nih.gov/23084240
- Dugel PU, Boyer DS, Antoszyk AN, Steinle NC, Varenhorst MP, Pearlman JA, Gillies MC, Finger RP, Baldwin ME, Leitch IM. Phase 1 Study of OPT-302 Inhibition of Vascular Endothelial Growth Factors C and D for Neovascular Age-Related Macular Degeneration. Ophthalmol Retina. 2020 Mar;4(3):250-263. pubmed.ncbi.nlm.nih.gov/31924544


